Share
1
item
избран
Получете достъп до изключителни дерматологични услуги, за да подобрите своите професионални знания: над 500 визуални материала за патологии, клинични случаи и експертни видеа
Възползвайте се от ценни функции: аудио слушане, материали за споделяне с вашите пациенти
Бъдете информирани за предстоящи събития и уебинари, последни научни публикации и продуктови иновации
Вече имате профил? Влезте тук
Reports written by Prof. Ivelina Yordanova (Dermatologist, Bulgaria)
Related topics
The 23rd Congress of the European Society for Pediatric Dermatology (ESPD) took place in the charming city of Košice, Slovakia, where the streets echoed with the lively tunes of chardash, transporting attendees to a beautiful fairy tale from times long past. The organizers of the congress, ESPD and the Slovak Dermatologic Society, led by Congress President Prof. Klara Martinaskova, expertly curated an atmosphere blending ancient culture and romance. The event featured high-tech presentations from experienced pediatric dermatologists across Europe, as well as enthusiastic young leaders and speakers from beyond Europe.
Speaker: Prof. Juliette Mazereeuw-Hautier
A significant advancement in the treatment of severe forms of ichthyosis was announced during one of the sessions.
Congenital ichthyoses are a rare group of monogenic diseases characterized by abnormal epidermal differentiation. Diagnosis is primarily based on clinical signs such as erythema, scaling, and pruritus. Conventional treatments typically involve local therapies with emollients and keratolytics, as well as systemic treatment with retinoids like Acitretin.
Recent research has revealed that inflammation in ichthyosis follows the IL23/Th17 axis. This prompts the question of whether biological products are effective in treating severe forms of the condition.
The presentation on "Biologicals in Ichthyosis" was delivered by Prof. Juliette Mazereeuw-Hautier, a dermatologist and university professor at Paul Sabatier University in Toulouse, France. She also serves as a hospital practitioner and heads a Referral Center for rare diseases and congenital ichthyoses. With an index of 30, Prof. Mazereeuw-Hautier has co-authored 164 publications receiving 4621 citations.
Her presentation discussed the accumulation of cases of severe ichthyosis treated with biological agents, prompting the question of their effectiveness in managing the condition. To answer this question, Prof. Juliette Mazereeuw-Hautier conducted a retrospective observational international study in 22 expert centers from 8 countries. The study was conducted from January 2018 to August 2023.
Inclusion criteria:
Exclusion criteria:
The study included 98 patients with severe forms of ichthyosis, comprising 49% women and 51% men. The patients were from various regions, with 63% from Europe, 34% from the USA, and 3% from Asia. The average age of the patients was 15.5 years, and the majority (72.5%) were Caucasian.
Patients are distributed by diagnosis as follows: Netherton syndrome (30%), Congenital ichthyosiform erythroderma (21%), and lamellar ichthyosis (13%). Most patients present a severe or very severe form of ichthyosis.
Regarding treatment, patients received topical therapy alongside biological products in 32% of cases, which included corticosteroids, calcineurin inhibitors, and keratolytics. Systemic treatment with oral retinoids, phototherapy, systemic corticosteroids, immunoglobulin, and methotrexate was administered in 24.5% of cases.
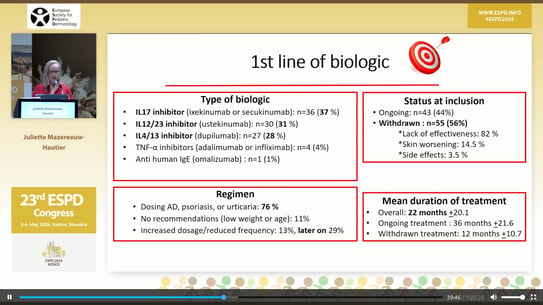
Out of the 98 patients, 81 received first-line biological therapy, while 16 received second-line therapy, and 1 patient received third-line therapy.
In first-line biologic treatment, patients received:
44% of the patients continued the treatment after the initial inclusion. The remaining 56% discontinued treatment with biological agents for the following reasons:
The mean duration of treatment was 22 months ± 21.6 months, with the longest treatment lasting 36 months ± 21.6 months. 76% of the patients followed the biological treatment regimen typical for psoriasis, urticaria, and atopic dermatitis.
The effectiveness of the treatment was evaluated by Investigator Global Assessment (IGA) score, revealing that worsening occurred in 8% of patients, with no improvement in 46%, slight improvement in 28%, and great improvement in 1% of patients with ichthyosis.
The medication's effect was further evaluated in terms of pruritus, erythema, and desquamation. Improvement in pruritus was observed in 73% of cases, reduction in erythema in 69% of cases, and improvement in desquamation in 58% of patients. Additionally, in Netherton's Syndrome, improvements in hair growth and quality of life were observed.
Side effects were noted in 16% of patients treated with biologics, with recurrent infections being the most common.
The question arises whether there are differences in response to biological treatment in subgroups of ichthyosis based on age, type of biological product, and form of ichthyosis. It was found that there were no age differences in response. While the type of biological product showed a trend for better effectiveness of IL12/23 inhibitors and IL4/13 inhibitors (56%) compared to IL17 inhibitors (33%), the form of ichthyosis was found to be important. Patients with Netherton syndrome responded best to IL12/23 inhibitors in 86% of cases. Netherton syndrome was identified as the best responder to biological treatment, contrasting with Congenital Ichthyosiform erythroderma, where the effect of biological products varied.
The conducted study has several limitations. Firstly, the limited number of patients in subgroups prevents statistical reliability of the analysis. Additionally, relapse occurs approximately 33 months after the end of treatment with a biological product. There are discrepancies in the data published in the literature regarding the effect of treatment of different forms of severe ichthyosis with biological products, with only 29 publications available, including a small total number of patients (69 in total) and short follow-up periods. There is a single study published in 2022 treated 20 patients with ichthyosis for 16 weeks with IL17 inhibitors, finding no statistically significant differences between those treated with a biological product and those treated with a placebo.
In conclusion, the results do not support the prescription of biologics in every patient with ichthyosis. Patients and their doctors should be aware that the effectiveness of biologic treatment cannot be predicted, but it is assumed that it will reduce erythema and inflammation of the skin in patients with Netherton syndrome. IL12/23 inhibitors may be more effective compared to IL17 inhibitors. However, the effect of slight improvement is usually transient and may worsen over time. Combining biologics with Acitretin is suitable given its effect on desquamation. It should also be noted that biological products are off-label for this treatment and are extremely expensive medications.
Speaker: Univ.-Prof. Dr. med. Ulrike Blume-Peytavi – Director Clinical Research Center for Hair and Skin Science Charité – Universitätsmedizin, Berlin
Trichotillomania, also known as hair-pulling, is a mental health disorder that causes kids to have an uncontrollable urge to pull out their hair. Pulling out hair from the head is most common. Some children also pull hair out from other parts of the body, including eyelashes, eyebrows, genitals, arms and legs. A lot of kids pull out their hair without knowing it.
Dermoscopy, a relatively recently available bedside tool, may be ideal for the examination of pediatric skin lesions, as it causes no physical discomfort or emotional distress. Dermoscopic features of trichotillomania include coiled hairs with frayed ends, short hairs with trichoptilosis (split ends) and flame hairs.
Pharmacotherapy for pediatric trichotillomania has shown mixed results. Selective serotonin reuptake inhibitors (SSRIs) are ineffective in reducing hair-pulling symptoms per se, while the opioid antagonist naltrexone and the atypical neuroleptic olanzapine show some efficacy. Cognitive-behavioral therapy and therapy with N-Acetylcysteine at a dosage of 2x1200 mg daily are well-tolerated, inexpensive, and easily accessible as over-the-counter options with a good safety profile. Clomipramine, the only FDA-approved drug for treating obsessive-compulsive disorder in ages 10 and older, is also utilized.
Short anagen hair (SAH) is a rare pediatric hair disorder characterized by a short anagen phase, leading to an inability to grow long scalp hair, and causing a negative psychological impact. A significant association has been found between the WNT10A genetic variant and SAH, particularly observed in individuals with light-colored hair and regression of the frontoparietal hairline. Rare WNT10A variants are associated with a phenotypic spectrum ranging from no clinical signs to severe ectodermal dysplasia. The presumed shared biological effect of WNT10A variants in SAH and MPHL (male pattern hair loss) is a shortening of the anagen phase.
Uncombable hair syndrome (UHS) is characterized by dry, frizzy hair that resists being combed flat. This condition typically develops in childhood, often between infancy and age 3, but can occur as late as age 12. Affected children typically have light-colored hair, described as blond or silvery with a glistening sheen. The hair grows out from the scalp in multiple directions rather than downward. Despite its appearance, the hair is not fragile or brittle, and it grows at a normal or slightly slower rate. Uncombable hair syndrome only affects scalp hair. It is important to investigate this syndrome as it may be an indicator of other, more serious health conditions.
Diseases such as Bork Stender Schmidt syndrome, ectodermal dysplasia, and angel-shaped phalangoepiphyseal dysplasia may be associated with UHS but, usually, cases are isolated. Most cases of UHS have a genetic origin. The syndrome has been found to be caused by genetic changes in the genes PADI3, TGM3, and TCHH. These 3 genes code for proteins that are involved in hair shaft formation. It is primarily an autosomal recessive inherited condition, although the NIH noted that autosomal dominant origin can also occur due to the involvement of multiple genes in hair formation. However, not all UHS cases exhibit mutations in these genes, leaving the cause of those patients' conditions unknown.
Alopecia areata (AA) is an immune-inflammatory disease characterized by non-scarring hair loss. Between 11 to 50% of cases occur in children, with the prevalence rate of pediatric AA slightly higher than that in adults. Since most pediatric patients with limited AA recover spontaneously, and the response to current therapies is unpredictable, evidence for the efficacy of treatments is mostly weak. Many parents discontinue treatment due to the lack of curative effect, common relapse, or concerns about side effects. Some patients, however, will develop recurrent symptoms. AA can affect all scalp skin.
Commonly accepted treatments for AA include intralesional corticosteroids, topical corticosteroids, Minoxidil, topical prostaglandin analogues, systemic corticosteroids, immunosuppressants, laser therapy, JAK inhibitors, biologics, phosphodiesterase 4 inhibitors, PRP therapy, Low-Dose IL-2 administration, statins, antihistamines, and microneedling. High-potency topical corticosteroids are the first-line treatment for pediatric patients.
Prof. Dr. med. Ulrike Blume-Peytavi presented a study on the efficacy of Coacillium, a new cutaneous, botanical solution composed of Allium cepa, Citrus limon, Theobroma cacao, and Paullinia cupana, which has shown promising results in impacting hair follicle cycling and endothelial cell activation, making it a potential treatment for moderate to severe alopecia areata in children and adolescents.
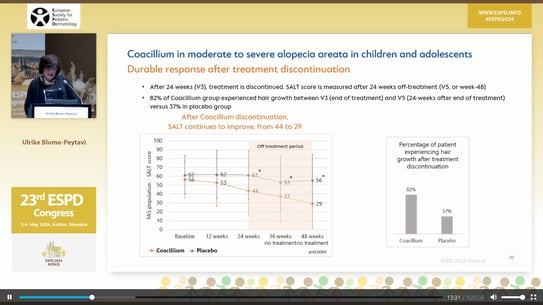
The study included 62 children and adolescents (mean age, 11 years; 45% girls) aged 2 to 18 years with severe (60%) or moderate (40%) AA. Each patient was randomly assigned to apply Coacillium solution 22.25% (n = 42) or placebo (n = 20) twice daily for 24 weeks, followed by a treatment-free period of another 24 weeks to evaluate relapse rates. The primary endpoint was a change in Severity of Alopecia Tool (SALT) scores, with 100 being the worst and 0 being the best, from baseline to week 24.
Results showed that after 24 weeks, patients treated with Coacillium saw a mean Severity of ALopecia Tool (SALT) score improvement of 22.87%, whereas placebo-treated patients regressed by 8%. During the treatment-free period, Coacillium-treated patients continued to improve, with SALT scores decreasing from 43.6 to 29. By week 48, 47% of Coacillium-treated patients had reached a SALT score of 20 or less compared with only 9.1% of placebo-treated patients (P = .0031). All adverse events were local, transient, mild, or moderate, apart from one severe transient eczema case. No serious adverse events were reported.
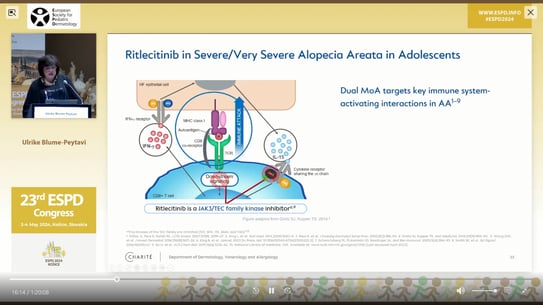
Prof. Dr. med. Ulrike Blume-Peytavi presented the efficacy and safety of a new oral, selective JAK3/TEC family kinase inhibitor called Ritlecitinib in patients with AA. She discussed the design and results of a randomized, double-blind, multicenter, phase 2b–3 trial conducted at 118 sites in 18 countries. The trial included patients aged 12 years and older with AA. Ritlecitinib is the first FDA and EMA approved drug for the treatment of children over 12 years old with AA.
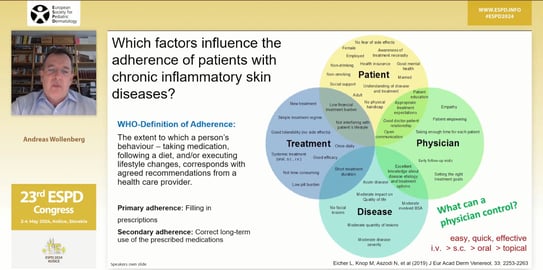
Speaker: Prof. Andreas Wollenberg (Munich, Germany)
In the final scientific session, Prof. Andreas Wollenberg from Munich presented his favorite topic – the holistic management of children and adolescents with mild to moderate atopic dermatitis (AD).
He emphasized why we focus on mild to moderate AD. He explained that most AD patients have reduced affected areas and mild lesions, and do not need systemic treatment. In this cohort, we need to avoid triggering factors, but we need to identify them first. We need to look at comorbidities, but they are not relevant in the mild to moderate population. Identifying infectious complications is very important because they can occur very easily even in moderate patients. Prevention of flare-ups is essential.
Patient characteristics and problems to be solved are different for every child: acute exacerbation of the disease requiring acute intervention; long-term chronic disease requiring immunomodulation; severe atopic disease with the need to prevent flare-ups.
According to Prof. Wollenberg, when we treat our pediatric patients with atopic dermatitis, we do not forget to look for and find contact allergies in them. In adult patients with AD, it is known that 1 in 4 patients has a contact allergy. In the last eight years, the incidence of contact allergy in children with AD has been the same as in adults with AD, and 1 in 3 children with AD has had some form of contact allergy. Preservatives and fragrances in emollients have also been shown to cause contact allergies in atopic children. That's why more emollients are now without fragrance and packaged in sterile tubes without preservatives.
Principles off treatment for AD are to stabilize the skin barrier, reduce inflammation in the skin and correct dysbiosis. If xerosis needs to be corrected, topical emollients must be applied immediately after showering. This should reduce epidermal water loss and increase the efficacy of topical corticosteroids. They can be combined with phototherapy, proactive treatment with topical corticosteroids and calcineurin inhibitors, and systemic treatment.
In children, topical corticosteroids should be used at the lowest strength, to lower the risk of developing iatrogenic Cushing's syndrome. Among the topical calcineurin inhibitors, tacrolimus is much more effective than pimecrolimus. JAK kinase inhibitors include JAK 1, JAK 2, JAK 3 and TYK2. The efficacy of the local JAK kinase inhibitor roxolitinibe is being investigated in atopic patients. It provides potent inhibition of the JAK 1 and JAK 2 receptors, and has a weaker effect on the TYK 2 receptor. There is currently a phase-III clinical trial in which roxolitinibe is being used topically in children with AD over 12 years of age.
Delgocitinib, which is used to treat hand eczema, is also used to treat children over 12 years of age with AD.
Crisaborole and roflumilast, from the phosphodiesterase inhibitor group, are also being used topically in cream form in clinical trials for children with AD. Roflumilast works much better than crisaborole.
It is recommended to implement a proactive treatment, i.e. intermittent application of anti-inflammatory treatment to the affected areas at the same time as emollients, which leads to longer remissions in patients.
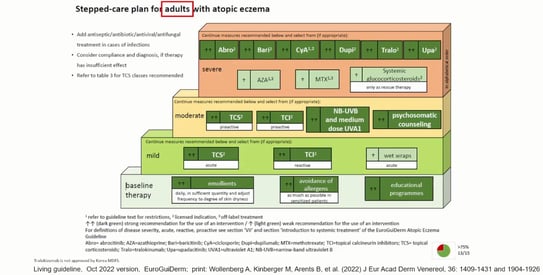
All systemic agents used in adults with AD can be used in children with AD over 12 years of age, except for dupilumab, which is used from 6 months of age. Finally, Prof. Wollenberg discussed how to get patients to adhere to their treatment, and explained the importance of education for patients with atopic dermatitis and their parents.

Prof. Ivelina Yordanova is pleased to share with you a series of photos from the ESDP Congress, where she was a reporter.




