Share
1
item
избран
Получете достъп до изключителни дерматологични услуги, за да подобрите своите професионални знания: над 500 визуални материала за патологии, клинични случаи и експертни видеа
Възползвайте се от ценни функции: аудио слушане, материали за споделяне с вашите пациенти
Бъдете информирани за предстоящи събития и уебинари, последни научни публикации и продуктови иновации
Вече имате профил? Влезте тук
Summary of the Bioderma Symposium held during the World Congress of Dermatology in Singapore in July 2023: "Ecobiology: Dialogue between skin barrier and its ecosystem" with Prof. Berardesca, Prof. Kabashima, Prof. Dréno and Dr. Fauverghe (Naos).
Related topics

Naos International Medical Director
Dear All,
I am very pleased to present you the sixth edition of Bioderma Updates Series dedicated to updates in Dermatology.
For 3 years now, Bioderma has been regularly organizing international events dedicated to Dermatology, for dermatologists and all healthcare professionals interested in Dermatology, always presented by renowned experts in their field.
In our approach to promote the development of knowledge in Dermatology, we have the pleasure to propose you this new publication, that is the summary of the Bioderma Symposium held during the World Congress of Dermatology in Singapore in July 2023: Ecobiology: Dialogue between skin barrier and its ecosystem with Enzo Berardesca from the USA, Kenji Kabashima from Japan, Brigitte Dréno from France and myself as speakers.
During this symposium, Enzo Berardesca presented: Skin barrier, an organ still in adaptation. Kenji Kabashima delivered a lecture about the environmental triggers and the skin barrier. The lecture of Brigitte Dréno, our chair, was about the skin microbiome, a new actor in cutaneous neurogenic inflammation. And, finally, I presented: Atopy, an ecobiological approach for a targeted skin care formula ecobiological approach of sun protection: to reinforce the natural mechanisms of the skin.
I wish you all an enjoyable, enriching and interesting reading.

Philip Frost Dept. of Dermatology University of Miami, Miami, USA
The human skin forms a protective barrier against the external environment and is our first line of defense against physical assaults (mechanical injury, UV-irradiation), microbial assaults (bacteria, fungus, virus), and chemical assaults (irritants, allergens). It also defines our outward appearance, protects our internal tissues and organs, and acts as a sensory interface. It has important homeostatic functions such as reducing water loss and contributing to thermoregulation of the body. Skin barrier disruption can lead to increased transepidermal water loss (TEWL) and dryness. The skin barrier is a living system adapting and evolving itself throughout our life. At birth, the skin barrier is not fully complete, and the beginning of postnatal life is a period for active adaptation and maturation of the cutaneous structure and functions(1). In infancy (first year after birth), skin structures and functions are similar to those of adults (excluding the inactive apocrine sweat glands).
Androgens may influence the barrier function, as testosterone perturbs epidermal permeability barrier homeostasis(2). Development of the skin barrier before bir th is increased by estrogens and decreased by testosterone. In experimental models, the inhibition of androgen production stimulated barrier recovery(2). While lower TEWL and increased skin reactivity were observed in some women as a function of the menstrual phase(3-5), no significant gender-differences in TEWL, hydration, pH or sebum were detected in other studies(6, 7). Skin repair is improved by estrogens; but repairing capabilities decrease after menopause(8). Increased pain sensitivity to noxious stimuli, increased chronic pain, and lower analgesic effects of drugs have been observed in women, as a function of the menstrual cycle(9).
With aging, and particularly in women after menopause, the skin becomes dryer and less flexible, with increased TEWL, fragmentation of collagen and elastin, and with fewer but larger corneocytes(10-12). As a result, skin barrier structure, permeability barrier function, epidermal calcium gradient, epidermal lipid synthesis and stratum corneum (SC) lipid processing, cytokine production and response after aggression, SC acidity, SC hydration, and antimicrobial barrier are changed or disturbed(13). Hydration becomes uneven in aged skin, and ethnic differences in skin dryness were found between Caucasian, African, American, Chinese, and Mexican women, with a higher percentage increase in Caucasian women(14, 15).
Epidermal dysfunction, compromised permeability homeostasis, reduced stratum corneum hydration and elevated skin surface pH predispose to the development of aging-associated cutaneous and extracutaneous disorders, including eczematous dermatitis, pruritus, and xerosis(16). Alterations in epidermal function can lead to the development of a chronic, low-grade systemic inflammation termed “inflammaging”, which is linked to the development of agingassociated systemic disorders(16).
As the epidermis forms a microbial, physical, chemical, immunological, and neuro-sensory barrier between the internal and external environment, it is important to consider different barriers rather than just a single phyical barrier(17).
The microbial barrier, or microbiome, is made of skin microorganisms which form the first barrier against the environment through various mechanisms of colonization resistance, including resource exclusion, direct inhibition, and/or interference(18). The skin microbiota also contributes to the differentiation and epithelialization of the physical skin barrier (Figure 1). Microbes boost the chemical barrier of the skin by producing lipases that digest sebum triglycerides to free fatty acids, which amplifies the acidity of skin and restricts colonization by transient and pathogenic species. Finally, microbes stimulate innate and adaptive immune defenses, such as the release of antimicrobial peptides, induction of neonatal tolerance, and development of protective immunity(19).
Figure 1. The microbial barrier influences the different skin barrier functions(19)
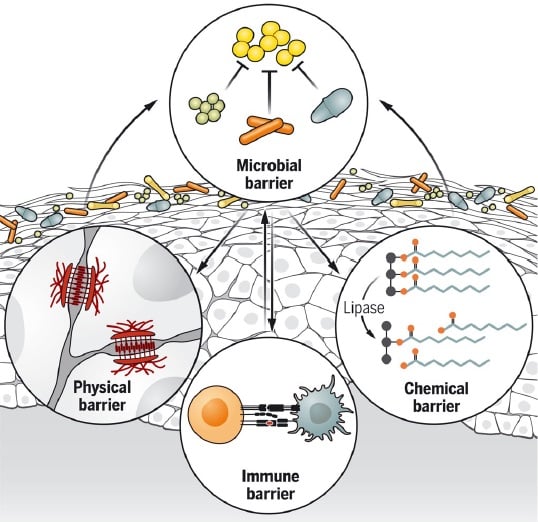
Structural cells such as keratinocytes, fibroblasts and adipocytes contribute to barrier immunity. Specialized immune cells present in the skin include mononuclear phagocytes, such as Langerhans cells, dermal macrophages and dermal dendritic cells, in addition to the resident memory T cells(20). Skin barrier immunity changes with age, and the skin immune composition becomes altered, with reduced Langerhans cells, decreased antigen-specific immunity and increased regulatory populations such as Foxp3+ regulatory T cells. Taken together, these alterations result in decreased skin barrier immunity in the elderly(20).
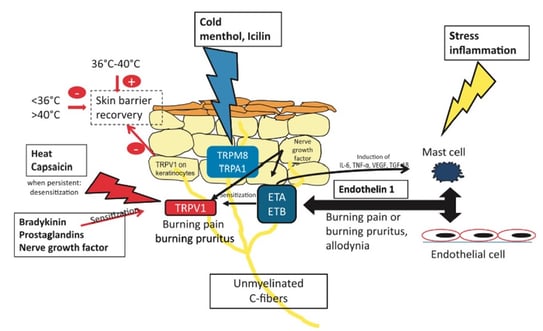
The neurosensory barrier is modulated by unmyelinated C-fibers that express endothelin receptors A and B (ETA and ETB), the capsaicin and heat receptor (TRPV1), and the cold receptors (TRPM8 and TRPA1) (Figure 2). Ligand stimulation induces a burning or itching sensation. Upon sensitization of these sensory neuroreceptors by inflammatory mediators, such as bradykinin, prostaglandins or nerve growth factor, receptor activation is enabled, which possibly underlies sensitive skin. Stress may induce mast cell degranulation, perpetuating the activation of ETA and ETB. Depending on the temperature, TRPV1 expressed in keratinocytes may increase or delay skin barrier recovery, possibly contributing to sensitive skin.
Figure 2. The neurosensory barrier(20)
The skin barrier is a living system adapting and evolving throughout our lifetime. There are several barriers, i.e., microbial, physical, chemical, immunological, and neuro-sensory, which act on different pathways of skin physiology and can modulate/interfere with local and systemic reactions. Finally, a healthy barrier is a healthy skin and a healthy body.

Kyoto University, Japan
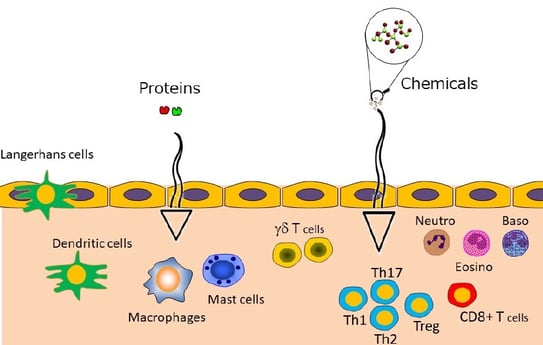
The skin is exposed to various pathogens including bacteria, viruses, proteins, and chemicals (Figure 1). The role of the physical barrier is to block pathogens and thus to prevent infection. Conversely, the role of the immunological barrier (Figure 2) is to eliminate proteins and chemicals and to prevent allergies. Allergens include proteins of biological origin and chemicals of non-biological origin. In healthy skin, the epidermis has two sets of physical barriers, the stratum corneum (SC) and the tight junctions (TJs)(1).These two barriers prevent the outside-in penetration of external antigens and the inside-out leakage of internal constituents. For skin appendages, such as hair follicles and sweat glands, TJs are the main barrier to block pathogens(2). The size threshold molecular weight, for the TJ barrier, is < 1.000 Da, thus, tacrolimus, gentamicin, steroids, fluorescein isothiocyanate (FITC), 2,4-dinitrofluorobenzene (DNFB), and water are able penetrate this barrier, but not big pathogens and pollens. Protein antigens are very large and have difficulty penetrating the normal skin. However, some small hapten FITC can easily penetrate the barrier(2).
Figure 1 External exposome factors(1)
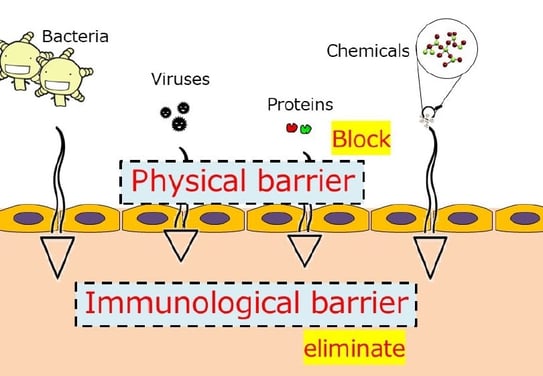
Figure 2 Immunological barrier of the skin(1)
Disruption of the skin barrier triggers inflammatory and immunological processes. Protein antigens induce delayed-type hypersensitivity (DTH), such as edematous erythema following insect bites. With chemicals, papules and vesicles observed in eczema are examples of contact hypersensitivity (CHS).
Once a pathogen has entered the skin, the immune response is induced, and dermal dendritic cells migrate more quickly than Langerhans cells to capture antigens. Moreover, neutrophils start to migrate rapidly inside the dermis, faster than T cells. After hapten application, dendritic cells (DCs) exhibit cluster formation around postcapillary venules. Effector T cells are activated within these DC clusters, called inducible skin-associated lymphoid tissue (iSALT), and produce cytokines(3).
Allergen exposure through the epidermis can initiate systemic allergy and predispose individuals to the development of one or more atopic diseases, including atopic dermatitis (AD or eczema), food allergy, asthma, and allergic rhinitis, via the so-called atopic march (Figure 3)(4). Two independent mutations in the gene encoding filaggrin (FLG) are associated with AD development(5). FLG is first produced as a polymer (profilaggrin), then becomes a monomer which aggregates to keratin fibers in epithelial cells. Upon degradation, FLG products account in part for the water-holding capacity and maintenance of acidic pH of the SC, both crucial for the epidermal barrier homoeostasis by regulating activity of multiple enzymes that control desquamation, lipid synthesis and inflammation(6). Thus, FLG is an essential protein for SC integrity. Skin barrier impairment in AD patients leads to an increase in transepidermal passage of chemical antigens, and then to chronic inflammation. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu, from a T helper cell type 1 to a T helper cell 2 profile(7).
Figure 3 Allergens trigger atopic dermatitis(4)
Protein antigens can penetrate the skin barrier in AD patients through small wounds, the protease activity of antigens, injections, and/or chronic inflammation. Allergy to latex, a protein sensitization occurring through small skin wounds, can sometimes lead to allergies to banana, avocado, kiwi, chestnuts, etc., called “latex-fruits syndrome”(8). Pollens have high protease activity, and pollen sensitization can result in pollen-food syndrome(9). A relationship between tick bites and sensitization to galactose-α-1,3-galactose (α-Gal), with the development of a red meat allergy as a secondary phenomenon has been shown(10). In Japan, jellyfish stings have been linked to sensitization to poly-γ-glutamic acid and, thus, allergy to natto, made with fermented soybeans, which can cause late-onset anaphylaxis(11).
Chronic inflammation is the most important mechanism of protein antigen sensitization in AD. Th2 cytokines have the potency to disrupt both the SC and the tight junction barriers(12, 13). Th2 cytokines can also cause itch by directly stimulating itch sensory neurons(14). Thymic stromal lymphopoietin (TSLP) is an itch mediator, and epithelial cells directly communicate to cutaneous sensory neurons via TSLP to promote itching(15, 16).
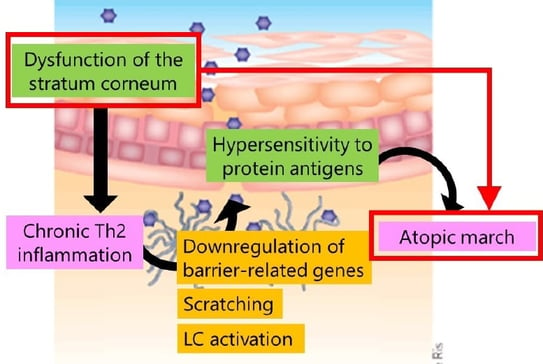
Once type 2 inflammation occurs, Langerhans cells start to migrate in the epidermis quite aggressively. Activated Langerhans cells extend their dendrites upward beyond the tight junction and can directly capture the external protein antigens(17). Thus, dysfunction of the stratum corneum can lead to chronic Th2 inflammation, which induces antigen-specific IgEs, leading to hypersensitivity to protein antigens in other organs. Downregulation of barrier-related genes directly induces scratching and Langerhans cell activation to further induce Type 2 inflammation. As dysfunction of the stratum corneum and hypersensitivity to protein antigens result in the atopic march, it is very important to maintain SC integrity. Gene mutations are responsible for the Netherton syndrome (SPINK5), SAM syndrome (desmoglein 1), and peeling skin syndrome (corneodesmosin), all involving disruption of the keratinocyte barrier function leading to AD-like skin inflammation, high serum IgE level, and high incidence of food allergy. In Japan, the daily application of moisturizer during the first 32 weeks of life has been shown to reduce the risk of AD/eczema in infants(18).
The skin barrier is composed of the physical (SC and TJs) and immunological barriers to stop environmental aggressors. In terms of atopic dermatitis, the filaggrin-related physical barrier (plus type 2 inflammation) is known to be important. Protein antigens have difficulties penetrating the intact skin barrier. Type 2 skin inflammation causes hypersensitivity to protein antigens. Finally, controlling skin inflammation is important to prevent the development of atopic march.

Nantes University, France
Various interactions exist between the skin microbiome and cytokine-triggered and neurogenic inflammations.
Of these 3 actors, the first one is the skin microbiome. As a collection of genomes of microorganisms (bacteria, fungi, viruses) residing on the skin surface, the microbiome plays an important role in human health(1).
The skin microbiome changes over the life course(2). In utero, the skin is sterile; the initial colonization of the skin occurring during delivery disappears by 6 weeks of age. Then, the skin-like profile is enriched with Staphylococcus and Corynebacterium species. At puberty, there is a major shift as sex hormones drive maturation of sebaceous glands, causing a proliferation of lipophilic Cutibacterium Acnes (C. acnes) and Malassezia species. In adults, the skin microbiome stability is remarkable, given the continuous disturbances induced by lifestyles, environment, etc. C. acnes and the pilosebaceous follicle are likely to be major stabilizers of this effect.
The microbiome is the guardian of skin barrier functions and the first barrier against the environment. It maintains skin homeostasis and controls the skin barrier epithelialization, with the aryl hydrocarbon receptor, a transcriptional factor, playing a major role. The microbiome boosts the skin chemical barrier, produces lipases, digests sebum triglycerides to free fatty acids, and amplifies skin acidity, thus restricting colonization by pathogenic bacteria. It modulates skin immunity and protects against pathogens, and releases extra cellular vesicles and antimicrobial peptides directly or by activating skin cells. For example, C. acnes, which represents >50% of bacterial species, produces propionic acid involved in skin odor, stimulates expression of β-defensin 2, and synthesizes vitamin B12 of which a deficiency is associated with hyperpigmentation. Staphylococcus hominis produces an antimicrobial peptide (AMP) with a unique inhibitory activity against S aureus. Staphylococcus lugdunensis produces lugdunin, an AMP-inducing production of the antimicrobial peptide LL-37. Micrococcus luteus degrades pollutants and isomerizes urocanic acid, which plays a role in UV protection. Staphylococcus Epidermis metabolizes sphingomyelin to produce ceramides(3, 4).
Bacteria interact among themselves by secreting extracellular vesicles (EVs). C. acnes constitutively releases extracellular EVs, increases proliferation of keratinocytes, modulates differentiation of keratinocytes with a decrease in keratin 10 and desmocollin 1 and an increase in filaggrin, and controls some commensal bacteria(5).
The second actor is cytokine-triggered inflammation. During activation of the innate immunity, the following pattern recognition receptors (PRRs) are expressed either at the surface of skin cells (keratinocytes) or intra-cellularly [toll-like receptors (TLRs), peroxisome-activated receptors (PARs), nod-like intracellular receptors (NLRs 1-3), and rig-like intracellular receptors (RLRs virus]. AMPs, the natural “antibiotic-like” peptides, are secreted by skin cells(1).
Loss of diversity of the microbiome leads to dysbiosis. In acne, dysbiosis can be linked to the loss of diversity of C. acnes phylotypes, with a predominant phylotype: IA1(6). Loss of diversity of C. acnes activates innate immunity(7). In an in vitro skin explant study assessing the impact on innate immune system (IIS) activation after C. acnes phylotype diversity loss, innate immune markers (IIMs) were significantly upregulated when incubated with phylotype IA1 alone, compared with the combination IA1 + II + III(7). The restoration of microbiome diversity helps to suppress inflammation via the down-regulation of innate immunity. The activation of innate immunity is associated with the secretion of cytokines. Anakinra (Kineret®), a recombinant and slightly modified version of the human interleukin 1 receptor antagonist protein, appears to be a promising option in the treatment of severe acne.
The 3rd actor, neurogenic inflammation, is a process involving the release of neuropeptides from sensory nerves, leading to inflammation(8). The activation of sensory nerve fibers triggers the release of neuropeptides, such as substance P (SP) and calcitonin ge ne - rel a te d p ept ide (CGRP) . Neuropeptides promote vasodilation, edema, and recruitment of immune cells, resulting in inflammation. Signals from the brain influence skin function, and vice versa, and the skin microbiome plays a crucial role in the skin-brain axis. Afferent fibers, unmyelinated C-fibers, myelin-type A fibers and autonomic nerve fibers are present in the skin with a dense distribution throughout all of its layers. The main neuropeptides released from these fibers include: substance P (SP), calcitonin gene-related peptide (CGRP), neuropeptide Y, natriuretic peptides (NP), and catecholamines (CT).
Interactions occur betweeen nerve fibers and bacteria. Bacillus cereus was the first bacteria to show the effect of SP on a cutaneous bacteria(9). B. cereus, a low-level skin bacterium, is responsible for acute cutaneous infections. In vitro, SP stimulates the cytotoxicity of B. cereus on HaCaT keratinocytes by acting on neurokinin 1 receptors (TACR1). Keratinocytes overproduce collagenase, and B. cereus overproduces superoxide dismutase.
Interactions also occur betweeen neuromediators and bacteria. In a healthy microbiome, bacteria interact strongly with each other via production of AMPs modulated by the degradation of enzymes such as peptidases. Neuromodulators modify the interactions between pathogen and commensal bacteria. SP and CGRP stimulate virulence in Staphylococcus aureus, increase adhesion to epidermal cells and production of virulence factors by Pseudomonas aeruginosa, and increase biofilm formation in S. epidermidis and P. fluorescens. Interactions also occur between SP, CGRP and S. epidermidis. The thermo-unstable ribosomal elongation factor (EfTu) is translocated to the S. epidermis surface through the mechanosensitive channels MscL. SP diffuses through the bacterial wall in S. epidermidis, links to EfTu, inducing the production of a biofilm. CGRP links to DnaK, leading to an increase in S epidermidis virulence. Finally SP and CGRP increase the virulence of S. epidermidis. S aureus is not sensitive to CGRP. Exposure of S. aureus and S. epidermidis to SP leads to increasing their cytotoxicity to keratinocytes through the overexpression of chemokines mRNAs, CCL5, CXCL1, and IL8. These results suggest that SP and CGRP modulate the activity of many diverse cutaneous Gram-positive bacteria on the skin.
Natriuretic peptides (NPs) and catecholamines (CT) are released by blood, capillary endothelial cells, C-tactile fibres and sympathetic C-fibres. NPs and CT mainly modulate S. aureus, S. epidermidis and C. acnes biofilm formation. For C. acnes, NP activity is different according to the phenotype. NP and CNP provide an important competitive advantage to C. acnes against S. aureus for biofilm formation. The activity of natriuretic peptides is dependant on temperature: at 37°C, the development of S. epidermidis biofilms is stimulated, whereas that of S. aureus is inhibited; at 33°C (skin temperature), the opposite effect can be observed. In the skin, NPs serve as thermostats to regulate biofilm formation activity by the different bacteria.
Bacteria are able to interfere with cutaneous physiology by producing molecules showing total or partial homology with skin. Neurotransmitters secrete molecules and are capable of acting as neurotransmitters. S. epidermidis and C. acnes are able to biosynthesize histamines causing pruritis. Corynebacteria synthesize glutamate, a neurotransmitter released in skin by primary sensory neurons.
Psychological stress induces alteration of the skin barrier function, increasing the severity of group A Streptococcus pyogenes infections. This alteration is due to an increase in endogenous glucocorticoids, which inhibits epidermal lipid synthesis by decreasing lamellar body secretion and decreases expression of defensins (cathelin-related AMP and β-defensin 3)(10). SP and its degrading enzymes are involved in the pathogenesis of acne, which in turn might partially explain the pathologic significance of neurogenic and psychogenic aspects in the disease process(11, 12). SP increases adhesion of both bacteria to the keratinocytes, secretion of enterotoxin C2 by S. aureus, and formation of biofilm by S. epidermidis(13).
Strains of the C. acnes type III lineage are associated with a skin condition called progressive macular hypomelanosis (PMH)(14).Skin commensal bacteria S. epidermidis and its by-product LTA promote melanocyte survival by inducing upregulation of TRAF1, CASP14, CASP5, and TP73. On the other hand, C. acnes can inhibit UVB-irradiated melanocyte survival by increasing apoptosis(15). In rosacea, cathelicidin processing is disturbed, resulting in peptide fragments causing inflammation, erythema and telangiectasias(16). Atopic dermatitis (AD) is a good exemple of combined cytokinic and neurogenic inflammation.
Antimicrobial peptides LL-37, β-defensins, and dermicidin are decreased in AD skin, favoring S. aureus colonization (role of IL-13, IL-31, IL-4). S. aureus grows poorly in acidic conditions (normal skin pH=5.3) but grows much better in AD, where pH is higher. C. acnes, which maintains the acidic pH of the skin by secreting propionic acid, is decreased in AD. Neurogenic inflammation via SP and natriuretic peptides increase the adherence of S. aureus to the stratum corneum. In addition, filaggrin deficiency (genetic or acquired from TH2 skewing), leads to irregular corneocytes facilitating S. aureus adherence. S. epidermidis and C. acnes produce histamine whose role in itching and pruritis is well known. The skin microbiome can control inflammation in AD. Proteases and phenol-soluble modulin-α (PSMα) secreted by S. aureus cause epidermal proteolysis and skin barrier damage in mice, which promotes inflammation and itching. S. hominis secretes peptides that inhibit PSMα produced by S. aureus(17). Inhibition of S. aureus activity by clinical S. hominis isolate correlates with the prevention of skin barrier damage and inflammation.
The skin microbiome opens the door to bacteriotherapy. Based on ex vivo findings of the modulation of inflammation with monoclonal antibodies to the cAMP factor 2, injection of the cAMP factor-targeted acne vaccine directly into acne lesions was proposed for potential use in the future(18). Acnes bacteriophages exist in the pilosebaceous unit, and a potential phage therapy targeting only C. acnes phylotype implicated in acne (1A1 mainly) has also been proposed for future research(19, 20). The rationale for a potential role of probiotics (live microorganisms) or prebiotics is based on their potential to correct dysbiosis. Antimicrobial peptides are produced by commensal bacteria of the microbiome.
The microbiome opens the door to ecobiological health science. By studying the relationship between living organisms and their environment (exposome and other organs), this interdisciplinary field combines the principles and methodologies from biology and environmental science. Through a comprehensive understanding of the permanently moving network that drives our body health and an ecobiological approach particularly adapted to the skin, the final goal of global health and harmony of the body could be reached.

Naos Medical Director, Lyon, France
Since its beginning, Bioderma has chosen an ecobiological approach to develop products that favor biomimetic ingredients and act on skin-own mechanisms and causes rather than on clinical signs alone. One of the objectives of Bioderma has been to develop a skin care formula that is specifically adapted to atopic dermatitis (AD).
In AD, an impaired skin barrier leads to allergen penetration, a decrease in natural moisturizing factor linked to filaggrin deficiency, an increase in TEWL linked to deficiency in the hydrolipidic film, and in an inadequate ceramide/cholesterol ratio. This generates skin inflammation and results in the clinical signs and symptoms of AD. Cutaneous bacterial dysbiosis is linked to the loss of diversity and an increase in the S. aureus biofilm, particularly during flare-ups(1, 2).
Both EU and US guidelines underline the importance of emollients in AD management(1, 3, 4). Current national and international recommendations suggest at least a once-daily application of moisturizers(5-7), and in appropriate quantity depending on the formulation(8, 9).
Lack of compliance is an issue as only one-third of atopic patients comply with their topical treatment, and half of the patients use less emollient than the recommended quantity(10, 11). Thus, it is necessary to propose emollients combining clinical efficacy, inflammation management, itching relief for quality of life (QOL), dermatological safety, optimal tolerance for all skin types, and adapted texture and sensoriality to encourage compliance.
Atoderm® Intensive baume was developed by Bioderma as an ecobiological approach to manage atopy. This balm contains LipigeniumTM, composed of biomimetic lipids and phytosphingosine, activates ceramide neosynthesis, restores filaggrin neosynthesis, and thus helps rebuild the skin barrier(12-14). Palmitoylethanolamide (PEA), Atoderm® Intensive baume’s second ingredient, is a biomimetic fatty acid known to regulate pruritus and improve skin comfort and quality of life of AD patients(15, 16). PEA provides relief from itching by acting on TSLP. In one study, after 3 weeks of use, there was a 70% reduction in itching score (p<0.0001), 94% of patients reported a decrease in their urge to scratch, and 88% of patients said that itching had stopped durably(17).
AD is characterized by a dysbiosis, with a sharp decline in microbial diversity. During AD flares, biofilm-growing Staphylococcus aureus emerges, in strict association with disease severity, as the major colonizer in AD skin lesions(18).
Atoderm® Intensive baume enhances skin barrier therapy, by limiting S. aureus adhesion on human corneocytes and inhibiting the formation of the S. aureus biofilm, thus preserving the skin microbiotic balance by acting on causes. Atoderm® Intensive baume decreases the amount of bacteria on the skin surface by 99.48%(19). Moreover, Atoderm® Intensive baume prevents flares(20). In that monocentric, double-blind, randomized, placebo-controlled study, 130 subjects (aged 6 months to 15 years) with moderate AD (SCORAD 15-40) were treated for 6 months with topical corticosteroids or tacro/picrolimus in combination with Atoderm® Intensive baume or a basic emollient (placebo). After 6 months , there were significant improvements in quality of life (QOL), with an 89% increase in QOL score, in SCORAD (51% improvement), and in PO-SCORAD (55% improvement)(20). In addition, more than ¾ of the patients had no relapse during the 6 months, and for the other 25% the time between flare-ups was 20 days or more. Finally, flare up severity was cut by half. Another study was conducted to assess the efficacy of Atoderm® Intensive baume on skin dryness during and after an outbreak of AD in subjects aged over 3 months and treated with topical corticosteroids(20). In this multicentric, prospective observational study, 125 subjects (>3 months old) with light to severe AD received 1 or 2 applications /day of Atoderm® Intensive baume on the face and body for 2 months(20). There were significant improvements in QOL, with an 85% decrease in pruritus and an 89% decrease in insomnia after 2 months. Dryness was reduced by 81% and scales by 92%(20). At the end of the study, 85% of the parents stated that they were no longer affected by their child’s skin problem, and 86% felt that it did not impact their own sleep any longer(20).
Atoderm® Intensive baume supports AD treatment during flare-ups. As a concomitant skin care, it helps the skin to rebuild its barrier and is extremely well tolerated without burning/tingling sensations. Between flare-ups, Atoderm® Intensive baume regulates itching and the inflammatory response, helps the skin strengthen its barrier, and improves the quality of life of patients and families. In both situations, it is very important to use the appropriate quantity of emollient. Atoderm® Intensive baume is an ecobiological approach that combines high efficacy, high tolerance and high compliance, for the well-being of the skin of both patients and their families.